MYLeukaemia technology

Our Technology
Inside our engineered human bone-marrow avatars, we add each patient’s leukaemia cells and challenge them with guideline-aligned and trial-ready regimens. High-content confocal / Flow cytometry readouts quantify true drug response, turning first-cycle trial-and-error into a 72-hour evidence-ranked plan that fits existing lab workflows.
In parallel, multiplex proteomics maps the signalling crosstalk between tumour, stroma and immune compartments - exposing microenvironment-driven resistance and surfacing actionable pathways for responder enrichment, MoA/biomarker discovery, and rational combination design.
Do you have any questions? Feel free to contact us.



How it Works
During routine diagnostics, the M.D. collects +5 mL extra peripheral/BM aspirate. Same-day courier brings the sample to our central lab, where we isolate viable leukaemia cells & load them into the MYLeukaemia Therapy Guidance Chipset - our bioengineered human bone-marrow avatar.
We challenge the blood cancer cells with 5 clinically used regimens, quantify Leukaemia cell response vs. healthy counterparts tolerance, and produce a tumour-board-ready heatmap that ranks most effective therapeutic options & flags QC. The result complements cytogenetics, flow, PCR and NGS, turning one-size fits all approach/trial-and-error into evidence-guided therapy selection.
For decisions perfectly tailored to your patient unique blood cancer profile.


1
> 100,000 patient leukaemia cells are isolated and integrated in a sophisticated MYLeukaemia Organ-on-Chip Chipset
Isolate Live Cells from Patients’ Blood






2
3
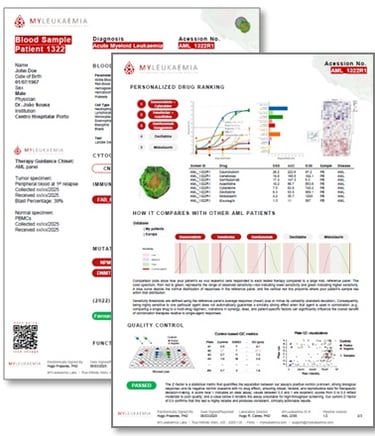

Patient-personalized Bone Marrow Avatar
Functional Precision Oncology sensitivity Testing
Drug testing for clinically used regimens ID's patients’ funcional AML drivers & rank drug sensitivity by FC
Patient-derived blasts colonize a 3D, vascularised Organ-on-Chip, recreating the patient’s bone marrow Leukemic niche within 24h.
MYLeukaemia Therapy Guidance Chipset is an R&D-stage functional precision oncology service (RUO/IVDR-aware). Not CE-IVD. Not a diagnostic. Clinical use restricted to IRB/ethics-approved pilots.

What Makes Our MYLeukaemia Chipset Unique


Designed for clinician-ready therapy ranking between cycles: measured, not guessed, and timely.




72-hour decision support
Unmatched native marrow-like niche bioengineering for clinically relevant, context-aware drug-response readouts.
Human Bone-Marrow avatars
Functional precision (ex vivo)
Functionally tests patient cancer cells beforehand to complement Standard-of-Care diagnostics (cytogenetics, flow, PCR, NGS) for truly personalized therapy choices.
Minimal-burden sampling
Just +5 mL added to routine peripheral or BM draws; no extra procedure.


Clear heatmap, ranked therapy options, QC flags; available clinical trials; in a simple & concise PDF report
Tumour-board-ready outputs




Pilot-ready & IVDR-aware
TRL4 with MVP on multiple AML cell lines; 20-patient pilot next; CE-IVD horizon ~24-36 months.

MYLeukaemia. All rights reserved. © 2025
Subscribe to our newsletter


No Spam. Just occasional product updates & events.
Company
About
Careers
News & Press
Contact
Campus da Penteada, 9020-105 Funchal, Portugal · myleukaemia@outlook.com


How to Join Us:
Start a Pilot
Request Study Design
Request Data Room
MYLeukaemia Therapy Guidance Chipset is an R&D-stage functional precision oncology service (RUO/IVDR-aware). Not CE-IVD. Not a diagnostic. Clinical use restricted to IRB/ethics-approved pilots.


