MYLeukaemia

About Us
Who are we: MYLeukaemia is an evidence-driven, Madeira-based spinoff advancing functional precision oncology for blood cancers - starting with AML & scaling to other blood cancers. Our Therapy Guidance Chipset recreates the bone-marrow niche (vascular and endosteal cues) on a microfluidic Organ-on-Chip, so we can observe how a patient’s cells respond to approved drug options before treatment begins. The aim: a ranked therapy report in ~72 hours that fits existing lab workflows.
Where we come from: The platform grew from two years of funded research at i3S / University of Porto. We’ve been recognized with early awards and selection to deep-tech programs, which helps - but our focus is disciplined execution, clinical proof, and patient benefit.
What’s next: We’re progressing from TRL-4 toward TRL-6 with prospective pilots. If you lead a haematology service, a reference lab network, or a pharma program in heme-oncology, we’d love to collaborate on validation, logistics integration, and clinical utility studies.
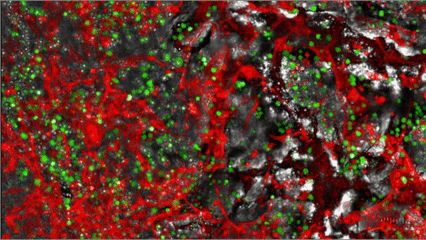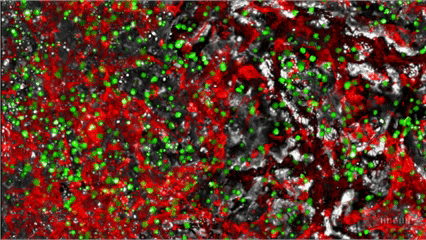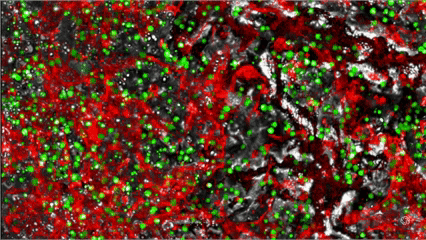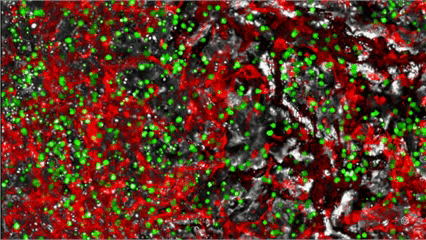

MYLeukaemia is under clinical validation and not yet a commercially available diagnostic. Results are provided for research/clinical-assessment use within applicable regulations.



CEO | Hugo Caires, PhD
Project Leader with +12 years of experience in Tissue Engineering, Cancer & Clinical Research in Leukaemia. +18 papers in top journals with +3000 citations.


CBSO | Hugo Prazeres, MBA, PhD
+30 papers in top journals, Co-founder of 3+ biotech companies, including Fetalix & BEAT Therapeutics. Consultant on CE marking and IVD Medical Devices.


COO | Diana Sousa, MSc
Pharmacist with +11 years of experience in sales, community healthcare & health economics, bridging clinical insight with real-world commercialization.
Complementary Expertise. One Founding Team.


CEO | Hugo Caires, PhD
Project Leader with +12 years of experience in Tissue Engineering, Cancer & Clinical Research in Leukaemia. +18 papers in top journals with +3000 citations.


CBSO | Hugo Prazeres, MBA, PhD
+30 papers in top journals, Co-founder of 3+ biotech companies, including Fetalix & BEAT Therapeutics. Consultant on CE marking and IVD Medical Devices.


COO | Diana Sousa, MSc
Pharmacist with +11 years of experience in sales, community healthcare & health economics, bridging clinical insight with real-world commercialization.
Strategic Guidance. Advisory Board & Mentors.

Early validation. Execution mode on.
From recognition on international stages to competitive grant funding and clinical alliances, MYLeukaemia has earned validation at every step. These achievements form the foundation for our next chapter: transforming personalised therapy selection for blood cancer patients.






+2
peer-reviewed articles published
+€60k
capital raised for R&D
+2
years of R&D in top institutions


Awards







MYLeukaemia. All rights reserved. © 2025
Subscribe to our newsletter


No Spam. Just occasional product updates & events.
Company
About
Careers
News & Press
Contact
Campus da Penteada, 9020-105 Funchal, Portugal · myleukaemia@outlook.com


How to Join Us:
Start a Pilot
Request Study Design
Request Data Room
MYLeukaemia Therapy Guidance Chipset is an R&D-stage functional precision oncology service (RUO/IVDR-aware). Not CE-IVD. Not a diagnostic. Clinical use restricted to IRB/ethics-approved pilots.



