Today +682,000 patients live with blood cancer in Europe.
+20%
Fail CR upon 1st induction
Costing ≥ €22 Billions to Europe’s public/private healthcare systems [1]
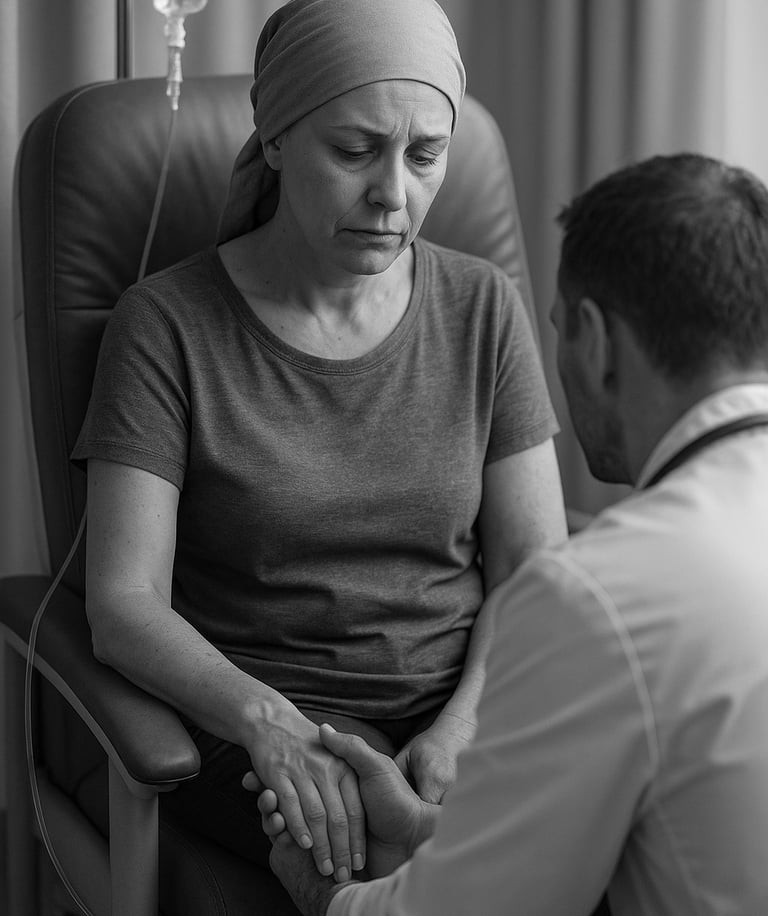
Nevertheless, outcomes in Leukaemias & Myeloma remain poor. Why? Lets take AML example _
Spotlight: Acute Myeloid Leukaemia
Primary-refractory median overall survival ≈6 months
+46%
Relapse within first 6 months
Early relapse halves chances of curative transplant eligibility
+70%
Won't survive beyond 5-year
Only ~15% reach 5-year survival after early relapse
Ordering Guidelines
When should I order?
Order when therapy decisions are unclear or for relapse cases requiring intensive therapy options.
What samples are accepted?
We accept various samples including bone marrow aspirate, with specific logistics for volume and anticoagulants.
How is the report structured?
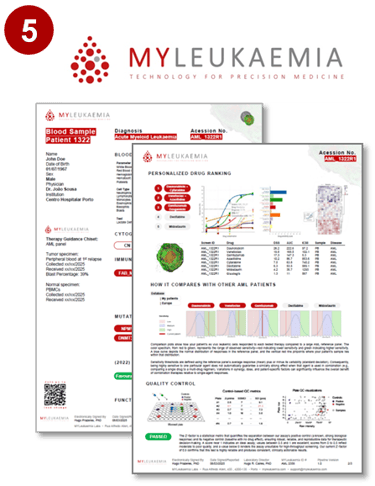
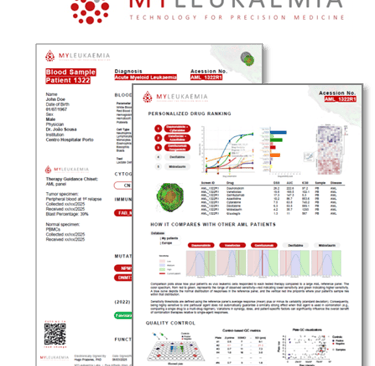
The report includes an interactive sensitivity heatmap, ranked options, QC flags, and downloadable PDF samples for easy access.
What is chain-of-custody?
Chain-of-custody ensures sample integrity during transport and handling.
How to read the report?
The report provides clear guidance on interpreting results and therapy options.
What integration pathways are available?
Integration pathways include EHR, LIS, and LIMS for seamless data management and reporting.
Innovative Solutions
Advanced therapies for personalized patient care.


Research Collaborations
Partnering for cutting-edge studies.
Clinical Insights
Driving informed treatment decisions.




Technology Integration
Optimizing workflows with innovative tech.
Patient-Centric Approach
Focused on individual patient outcomes.
→
→
→
→
4. LEAD THE CHANGE
We help clinicians, patients, and industry teams explore therapy options using ex vivo evidence: safely, transparently, and with rigorous governance.
For Haematologists
Run an investigator-led pilot inside routine workflows. We supply kits, SOPs and logistics; patient-derived cells are tested in our engineered bone-marrow avatars. You receive a concise pilot report designed to support tumor-board discussion (mock preview available). Target TAT from sample receipt: ≤72h. Investigational use only (not CE-IVD).
For Patients & Caretakers
We are now for investigational use only. However, you can partner with us via your Haematology team: join our Patient Advisory Circle, give usability feedback, or participate in ethics-approved pilots when available. Always optional, privacy-first, withdrawable anytime. Your consented input helps us shape tomorrows' fully personalised, patient-shared decision-making.
Use ex vivo patient biology in haem-onc to triage trial candidates, prioritise combos, and generate responder-enrichment hypotheses. Define cohorts, endpoints, QC, and data packages; align timelines in a study-design session—leveraging NAMs-aligned, near-human platforms supporting FDA Modernization Act 2.0 for drug discovery/validation.
For Pharmaceutical partners




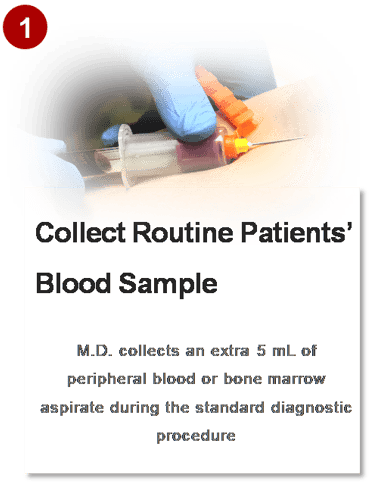
3. HOW IT WORKS
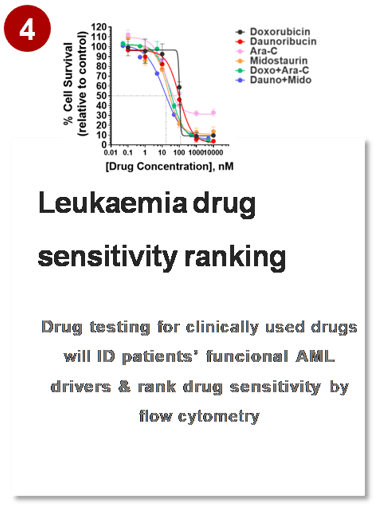
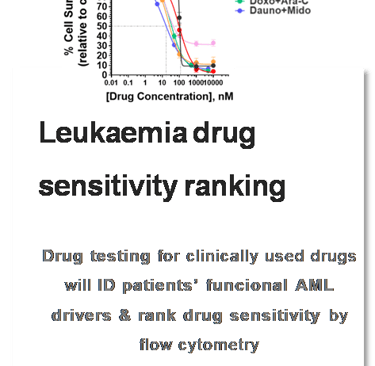

MYLeukaemia. All rights reserved. © 2025
Subscribe to our newsletter


No Spam. Just occasional product updates & events.
Company
About
Careers
News & Press
Contact
Campus da Penteada, 9020-105 Funchal, Portugal · myleukaemia@outlook.com


How to Join Us:
Start a Pilot
Request Study Design
Request Data Room
MYLeukaemia Therapy Guidance Chipset is an R&D-stage functional precision oncology service (RUO/IVDR-aware). Not CE-IVD. Not a diagnostic. Clinical use restricted to IRB/ethics-approved pilots.


