72-hour Therapy Guidance from Bone-Marrow-on-a-Chip Avatars
We engineer miniaturized human bone-marrow avatars, add each patient’s blood-cancer cells, and test approved and trial-ready therapies to deliver a 72-hour, evidence-ranked plan - guiding more effective, less-toxic care in blood cancers while helping clinical trials pre-select likely responders for cleaner, faster readouts.

Our Product
Meet the MYLeukaemia Therapy Guidance Chipset: an organ-on-chip (OoC) that recreates the bone-marrow niche to capture how each patient’s cells actually respond to available therapies turning trial-and-error into evidence-guided therapy selection in just 72h.
Discover our innovative marrow-avatar platform.


How it Works
During routine diagnostics, the M.D. collects +5 mL extra peripheral/BM aspirate. Same-day courier brings the sample to our central lab, where we isolate viable leukaemia cells & load them into the MYLeukaemia Therapy Guidance Chipset - our bioengineered human bone-marrow avatar.
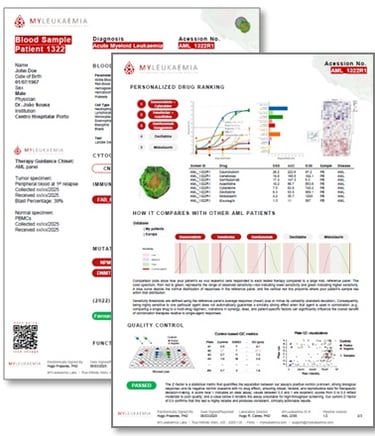
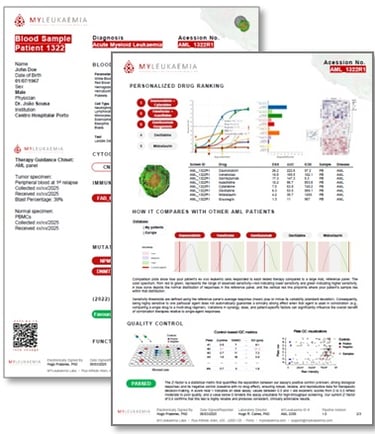
We challenge the blood cancer cells with 5 clinically used regimens, quantify Leukaemia cell response vs. healthy counterparts tolerance, and produce a tumour-board-ready heatmap that ranks most effective therapeutic options & flags QC. The result complements cytogenetics, flow, PCR and NGS, turning one-size fits all approach/trial-and-error into evidence-guided therapy selection.
For decisions perfectly tailored to your patient unique blood cancer profile.


1
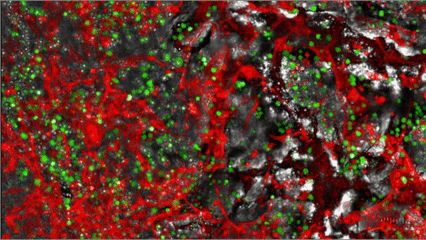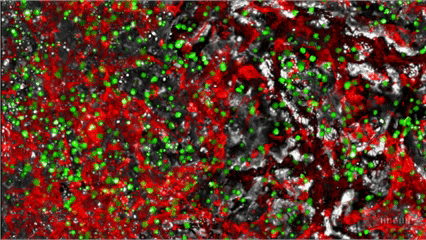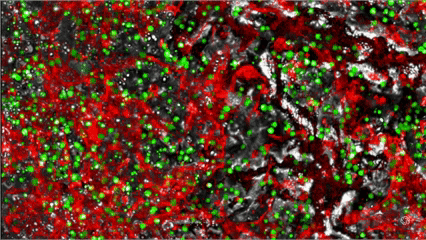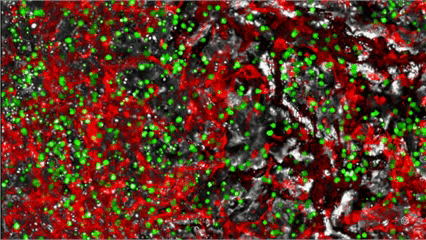
> 100,000 patient leukaemia cells are isolated and integrated in a sophisticated MYLeukaemia Organ-on-Chip Chipset
Isolate Live Cells from Patients’ Blood






2
3
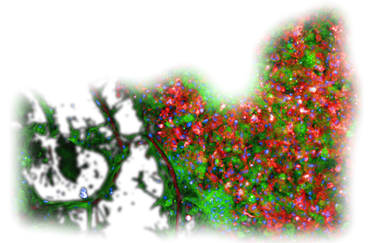
Patient-personalized Bone Marrow Avatar
Functional Precision Oncology sensitivity Testing
Drug testing for clinically used regimens ID's patients’ funcional AML drivers & rank drug sensitivity by FC
Patient-derived blasts colonize a 3D, vascularised Organ-on-Chip, recreating the patient’s bone marrow Leukemic niche within 24h.
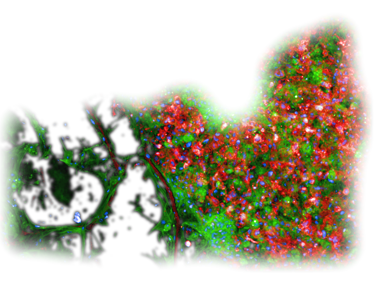
MYLeukaemia Therapy Guidance Chipset is an R&D-stage functional precision oncology service (RUO/IVDR-aware). Not CE-IVD. Not a diagnostic. Clinical use restricted to IRB/ethics-approved pilots.

What Makes Our MYLeukaemia Chipset Unique


Designed for clinician-ready therapy ranking between cycles: measured, not guessed, and timely.




72-hour decision support
Unmatched native marrow-like niche bioengineering for clinically relevant, context-aware drug-response readouts.
Human Bone-Marrow avatars
Functional precision (ex vivo)
Functionally tests patient cancer cells beforehand to complement Standard-of-Care diagnostics (cytogenetics, flow, PCR, NGS) for truly personalized therapy choices.
Minimal-burden sampling
Just +5 mL added to routine peripheral or BM draws; no extra procedure.


Clear heatmap, ranked therapy options, QC flags; available clinical trials; in a simple & concise PDF report
Tumour-board-ready outputs




Pilot-ready & IVDR-aware
TRL4 with MVP on multiple AML cell lines; 20-patient pilot next; CE-IVD horizon ~24-36 months.


CEO | Hugo Caires, PhD
Project Leader with +12 years of experience in Tissue Engineering, Cancer & Clinical Research in Leukaemia. +18 papers in top journals with +3000 citations.


CBSO | Hugo Prazeres, MBA, PhD
+30 papers in top journals, Co-founder of 3+ biotech companies, including Fetalix & BEAT Therapeutics. Consultant on CE marking and IVD Medical Devices.


COO | Diana Sousa, MSc
Pharmacist with +11 years of experience in sales, community healthcare & health economics, bridging clinical insight with real-world commercialization.
Complementary Expertise. One Founding Team.

Our trusted partners & supporters






From recognition on international stages to competitive grant funding and clinical alliances, MYLeukaemia has earned validation at every step. These achievements form the foundation for our next chapter: transforming personalised therapy selection for blood cancer patients.






+2
peer-reviewed articles published
+€60k
capital raised for R&D
+2
years of R&D in top institutions


Awards





Early validation. Execution mode on.

Follow us on LinkedIN
MYLeukaemia
24 September, 2025
We’re sharing APCL’s clear explainer on Leukaemias. We stand with patients, families, and clinicians - and with the science still ahead. At MYLeukaemia, our mission is to bring functional precision science closer to hospital decision-making, so more people get the right therapy, sooner.






MYLeukaemia
17 July, 2025
We made the top 7. 🧬
Out of many groundbreaking startups across Europe, MYLeukaemia Project is heading to the finals of the EP PerMed Venture Creator.
Still processing (...)
MYLeukaemia
13 May, 2025
He was in his 30s.
Same age as me.
I didn’t know his name.
I just knew his blood.
He was my first ever blood cancer case.
Intermediate risk. Good initial response.
Everything said it should work (...)

MYLeukaemia. All rights reserved. © 2025
Subscribe to our newsletter


No Spam. Just occasional product updates & events.
Company
About
Careers
News & Press
Contact
Campus da Penteada, 9020-105 Funchal, Portugal · myleukaemia@outlook.com


How to Join Us:
Start a Pilot
Request Study Design
Request Data Room
MYLeukaemia Therapy Guidance Chipset is an R&D-stage functional precision oncology service (RUO/IVDR-aware). Not CE-IVD. Not a diagnostic. Clinical use restricted to IRB/ethics-approved pilots.








